How Humidity Control Protects Buildings, Products, and People

Monitoring humidity directly impacts various aspects, including health, comfort, and industrial processes.
Monitoring humidity is crucial for several reasons, as it directly impacts various aspects of daily life, health, comfort, and even industries.
For instance, humidity monitoring improves respiratory health and comfort by maintaining an optimal indoor humidity level (typically between 30% and 50%), which helps the body regulate temperature through sweat evaporation.
Humidity control also preserves buildings and prevents damage by stopping condensation on walls and windows, which can cause structural damage and mold over time. In agriculture and greenhouses, monitoring humidity is essential to ensure proper growing conditions.
Industries such as pharmaceuticals, food production, and electronics rely on precise humidity control. High humidity can spoil food, degrade pharmaceutical products, and damage sensitive electronics. Also, in museums and libraries humidity should be monitored to preserve paintings, books, and historical artifacts, which can degrade with fluctuating moisture levels.
In many industrial processes, especially in textiles, and paper production, controlling and monitoring humidity is vital to ensure product quality and reduce waste, as improper humidity can lead to defective products, shrinkage, or material instability.
Given its importance across so many applications, let's first understand what humidity is.
Physics of Water Vapor
Humidity describes the quantity of water vapor in a gas. There are different ways to express humidity, e.g. relative humidity, absolute humidity, dew point temperature or mixing ratio. Consider a closed chamber with liquid water as shown in the next illustration.
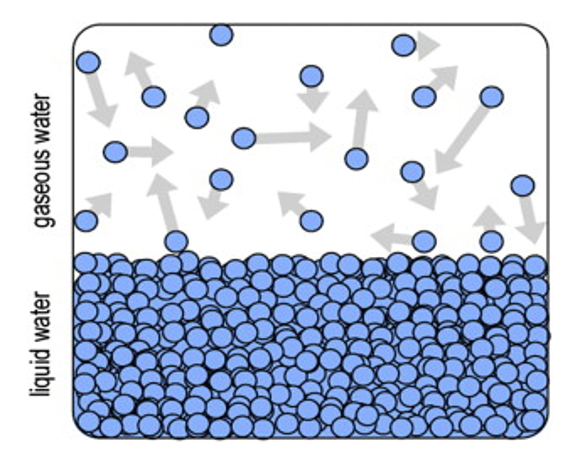
Closed chamber with liquid water and gaseous water particles (vapor).
The average kinetic energy, and the impulse of the water particles in the liquid water, is managed by temperature. Some particles have higher energy than the average and can escape from the liquid form – also known as evaporation above liquid.
Since the gaseous particles bounce around the closed chamber some of them will hit the liquid water surface and may be trapped in the liquid again. Eventually, equilibrium is reached, where the number of water particles evaporating equals those returning to the liquid.
When the equilibrium is achieved, the number of gaseous water particles remains statistically constant.
Since the gaseous water particles hit the wall of the closed chamber in the image above, a pressure e (i.e. evaporation, and it is used for partial water pressure only) is exerted to the wall. In equilibrium, this pressure is called saturated vapor pressure ew above water, which can be expressed by the Clapeyron relation:

where LV is the latent heat, T is the absolute temperature (in Kelvin), and ΔV is the volume change of the phase transition. Since the specific volume of the liquid is much smaller than the one of gas, and assuming the vapor gas is ideal, equation (1) can be approximated to the Clausius-Clapeyron equation:

where RV is the water vapor gas constant. Solving differential equation (2) leads to the Magnus formula for the saturated vapor pressure:
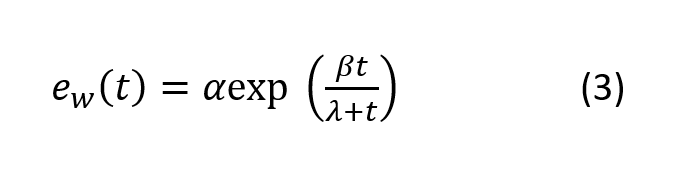
Where t is the temperature (in degree Celsius) and α, β and λ are the Manus parameters (see table below).
| Conditions | t (°C) | α (hPa) | β | λ (°C) |
| Above water | −45 to 60 | 6.112 | 17.62 | 243.12 |
As an example, the saturated vapor pressure above water is represented in image below. Between temperatures of −45°C and 60°C, equation (3) has an uncertainty of less than ±0.6% at 95% confidence level. If more accuracy is required, equations for saturated vapor pressure often led to implicit functions that can only be solved numerically.
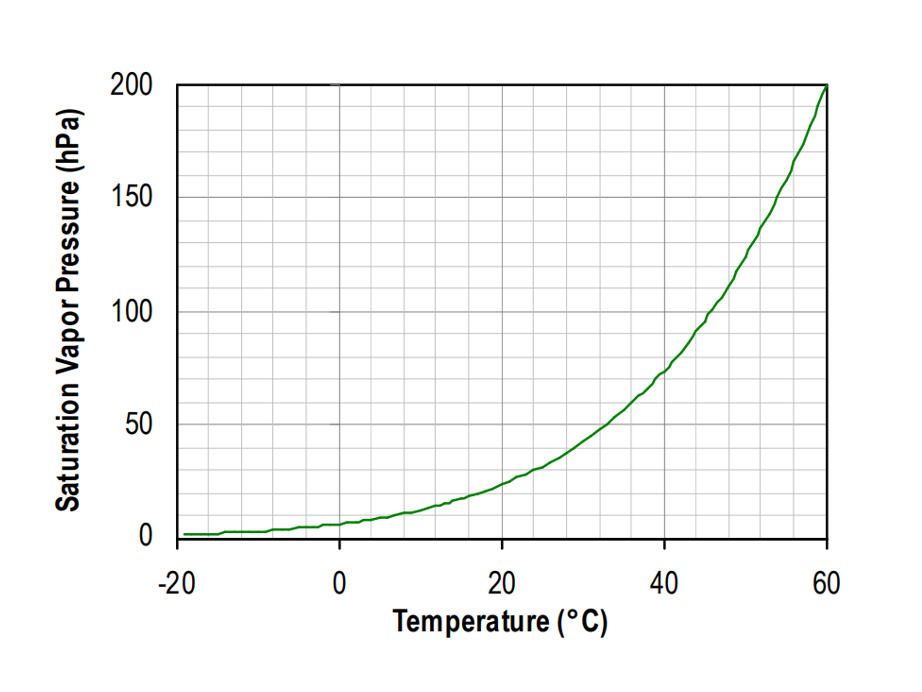
Saturated vapor pressure above water variation with temperature.
Relative Humidity
Relative humidity above water UW is mostly used for measurement purposes. It is defined as the ratio of the partial vapor pressure in air e to the saturated vapor pressure at a given temperature t, ew (t).
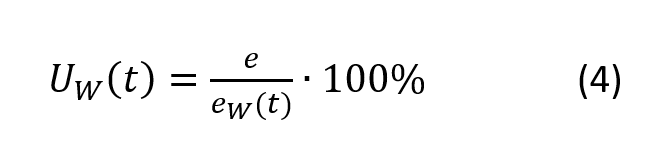
Thus, when equilibrium is achieved in the closed chamber, the relative humidity reaches 100%.
For example, with a relative humidity of about 90% at room temperature, a 1°C temperature change can cause a variation of up to –5%.
Dew Point Temperature
Consider a quantity of air with constant number of water particles (i.e. no condensation or evaporation) at a certain temperature t and relative humidity U < 100%. The dew point temperature td is defined as the temperature required to this quantity of air cooled down such that, at constant pressure, condensation occurs (U = 100%).
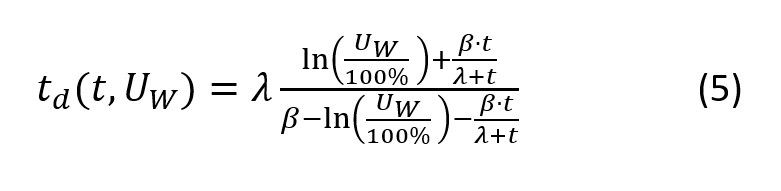
Absolute Humidity
Absolute humidity dV is defined by the mass of water vapor mH2O per humid air volume V and can be expressed as
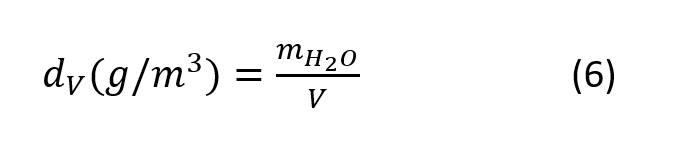
Applying the ideal gas law to partial water pressure e (eV=nRT),where V is the volume, n the number of water particles in mol, R the universal gas constant (8.314472 J mol-1 K-1) and T the absolute temperature, and applying the molar weight of water MH2O=18.0 g/mol, the following equation results
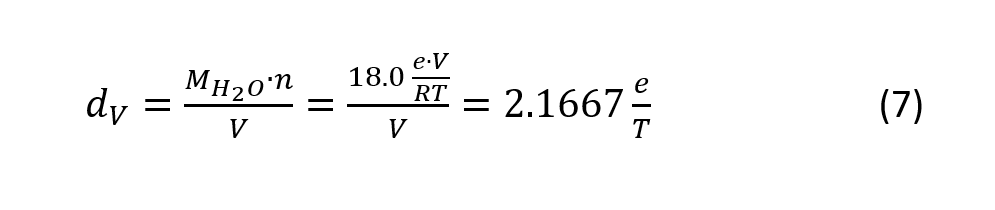
where T is the temperature in Kelvin, and e is the partial water pressure in Pa.
Mixing Ratio
Mixing Ratio r is the ratio of the mass of water vapor and the mass of dry air. Using the molar mass of dry air (29.0 g/mol) and water vapor (18.0 g/mol), the mixing ration can be expressed as
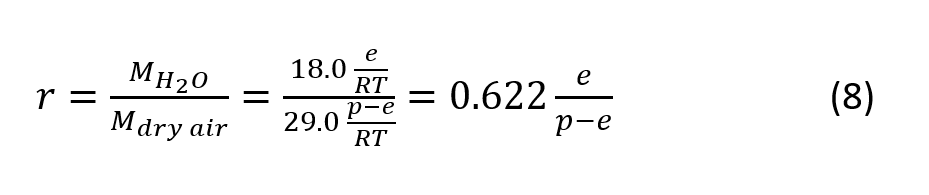
Where p is the total barometric pressure.
In summary, monitoring and controlling humidity ensures a balance that protects health, infrastructures, and materials while optimizing comfort and efficiency in various settings.
Tekon Electronics integrates humidity sensors to monitor the relative humidity levels in the DUOS inAir, DUOS inHygrotemp and DUOS Hygrotemp equipments. Also, these transmitters also monitor the external temperature.
Tekon Electronics remains at the forefront of innovation, delivering competitive and unique IoT solutions.
Get in touch and join us on the journey to a smarter, more connected future.